Complementarity-determining region
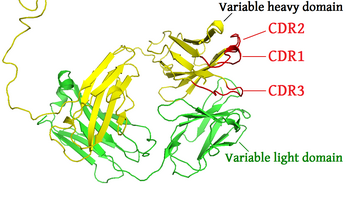
Complementarity-determining regions (CDRs) are polypeptide segments of the variable chains in immunoglobulins (antibodies) and T cell receptors, generated by B-cells and T-cells respectively.
As the most variable parts of the molecules, CDRs are crucial to the diversity of antigen specificities generated by lymphocytes.
[1] There are three CDRs (CDR1, CDR2 and CDR3), arranged non-consecutively, on the amino acid sequence of a variable domain of an antigen receptor.
Sixty CDRs can be found on a pentameric IgM molecule, which is composed of five antibodies and has increased avidity as a result of the collective affinity of all antigen-binding sites combined.
The diversification of the CDR-H3 will ultimately give antibodies their specificity, and ability to recognize antigens[5] Other factors contribute to the antibody-antigen interaction, including amino acid residues.