CRISPR gene editing
Knock-out mutations caused by CRISPR-Cas9 result from the repair of the double-stranded break by means of non-homologous end joining (NHEJ) or POLQ/polymerase theta-mediated end-joining (TMEJ).
[12] In 2023, the first drug making use of CRISPR gene editing, Casgevy, was approved for use in the United Kingdom, to cure sickle-cell disease and beta thalassemia.
[15] In the early 2000s, German researchers began developing zinc finger nucleases (ZFNs), synthetic proteins whose DNA-binding domains enable them to create double-stranded breaks in DNA at specific points.
CRISPRs are much easier to design because the process requires synthesizing only a short RNA sequence, a procedure that is already widely used for many other molecular biology techniques (e.g. creating oligonucleotide primers).
[17][18][19] In 2005, Alexander Bolotin at the French National Institute for Agricultural Research (INRA) discovered a CRISPR locus that contained novel Cas genes, significantly one that encoded a large protein known as Cas9.
Although Charpentier and Doudna (referred to as CVC) were credited for the conception of CRISPR, the Broad Institute was the first to achieve a "reduction to practice" according to patent judges Sally Gardner Lane, James T. Moore and Deborah Katz.
[46] In December 2021, it was reported that the first CRISPR-gene-edited marine animal/seafood and second set of CRISPR-edited food has gone on public sale in Japan: two fish of which one species grows to twice the size of natural specimens due to disruption of leptin, which controls appetite, and the other grows to 1.2 times the natural average size with the same amount of food due to disabled myostatin, which inhibits muscle growth.
"Almost half of the 32 participants from Germany who are scientists demonstrated constant choices, while the majority showed increased willingness to buy CRISPR tomatoes, mostly non-scientists.
[56] In November 2023, the United Kingdom's Medicines and Healthcare products Regulatory Agency (MHRA) became the first in the world to approve the use of the first drug based on CRISPR gene editing, Casgevy, to treat sickle-cell anemia and beta thalassemia.
Combined transient inhibition of NHEJ and TMEJ by a small molecule and siRNAs can increase HDR efficiency to up to 93% and simultaneously prevent off-target editing.
[90][91] Research has also been conducted in engineering new Cas9 proteins, including some that partially replace RNA nucleotides in crRNA with DNA and a structure-guided Cas9 mutant generating procedure that all had reduced off-target effects.
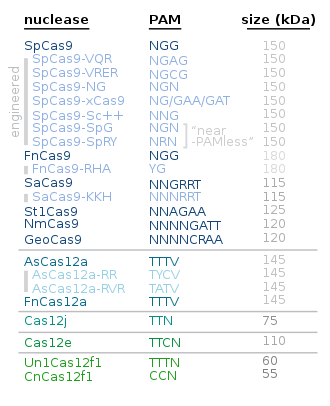
[120] The clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9 system is a gene-editing technology that can induce double-strand breaks (DSBs) anywhere guide ribonucleic acids (gRNA) can bind with the protospacer adjacent motif (PAM) sequence.
Knock-out libraries are created in a way to achieve equal representation and performance across all expressed gRNAs and carry an antibiotic or fluorescent selection marker that can be used to recover transduced cells.
[134] Successful in vivo genome editing using CRISPR-Cas9 has been shown in numerous model organisms, including Escherichia coli,[135] Saccharomyces cerevisiae,[136][137] Candida albicans, Methanosarcina acetivorans,[138][139] Caenorhabditis elegans,[140] Arabidopsis spp.,[141] Danio rerio,[142] and Mus musculus.
[152] For instance, when applied to human pluripotent stem cells, CRISPR has been used to introduce targeted mutations in genes relevant to polycystic kidney disease (PKD) and focal segmental glomerulosclerosis (FSGS).
[171] CRISPR may also have applications in tissue engineering and regenerative medicine, such as by creating human blood vessels that lack expression of MHC class II proteins, which often cause transplant rejection.
[172] In addition, clinical trials to cure beta thalassemia and sickle cell disease in human patients using CRISPR-Cas9 technology have shown promising results.
[173][174] In December 2023, the US Food and Drug Administration (FDA) approved the first cell-based gene therapies for treating sickle cell disease, Casgevy and Lyfgenia.
[175] Nevertheless, there remains a few limitations of the technology's use in gene therapy: the relatively high frequency of off-target effect, the requirement for a PAM sequence near the target site, p53 mediated apoptosis by CRISPR-induced double-strand breaks and immunogenic toxicity due to the delivery system typically by virus.
On November 17, 2021 CRISPR therapeutics and ViaCyte announced that the Canadian medical agency had approved their request for a clinical trial for VCTX210, a CRISPR-edited stem cell therapy designed to treat type 1 diabetes.
[180] Initial results in the treatment and cure of HIV have been rather successful, in 2021 9 out of 23 humanized mice treated with a combination of anti-retrovirals and CRISPR/Cas-9 had the virus become undetectable, even after the usual rebound period.
Anti-herpesvirus CRISPRs have promising applications such as removing cancer-causing EBV from tumor cells, helping rid donated organs for immunocompromised patients of viral invaders, or preventing cold sore outbreaks and recurrent eye infections by blocking HSV-1 reactivation.
[205] The CRISPR treatment for LCA10 (the most common variant of Leber congenital amaurosis which is the leading cause of inherited childhood blindness) modifies the patient's defective photoreceptor gene.
[180] In November 2022, Editas reported that 20% of the patients treated had significant improvements, but also announced that the resulting target population was too small to support continued independent development.
[citation needed] This disease comes under genetic disorders which are caused by mutation occurring in the structure of hemoglobin or due to substitution of different amino acids in globin chains.
[216] CRISPR has been used to develop higher quality crops, including improvements to physical appearance, flavor and aroma, texture, shelf life, and nutritional content.
The researchers reported it could be used to deliver genes as long as 36,000 DNA base pairs to several types of human cells and thereby potentially for treating diseases caused by a large number of mutations.
[280] Prime editing does not cut the double-stranded DNA but instead uses the CRISPR targeting apparatus to shuttle an additional enzyme to a desired sequence, where it converts a single nucleotide into another.
They said "scientists should avoid even attempting, in lax jurisdictions, germline genome modification for clinical application in humans" until the full implications "are discussed among scientific and governmental organizations".
[287] In April 2015, Chinese scientists reported results of an attempt to alter the DNA of non-viable human embryos using CRISPR to correct a mutation that causes beta thalassemia, a lethal heritable disorder.