CYP17A1
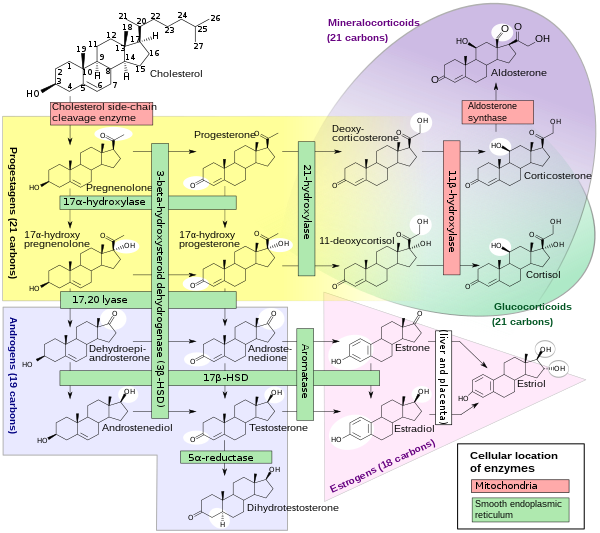
[6][7] It has both 17α-hydroxylase and 17,20-lyase activities, and is a key enzyme in the steroidogenic pathway that produces progestins, mineralocorticoids, glucocorticoids, androgens, and estrogens.
As an enzyme, CYP17A1 possesses an active site that associates with a heme prosthetic group to catalyze biosynthetic reactions.
Proteins in this family are monooxygenases that catalyze synthesis of cholesterol, steroids and other lipids and are involved in drug metabolism.
Overall, CYP17A1 is an important target for inhibition in the treatment of prostate cancer because it produces androgen that is required for tumor cell growth.
Although generally anovulatory, there are some case reports of women with 17α-hydroxylase deficiency who underwent spontaneous menarche with cyclic menses.
[9] In 2011, the FDA approved the CYP17A1 inhibitor, abiraterone, which contains a steroidal scaffold that is similar to the endogenous CYP17A1 substrates, with prednisone for the treatment of castration-resistant prostate cancer.
Abiraterone is structurally similar to the substrates of other cytochrome P450 enzymes involved in steroidogenesis, and interference can pose a liability in terms of side effects.
[28] The drug abiraterone acetate, which is used to treat castration-resistant prostate cancer, blocks the biosynthesis of androgens by inhibiting the CYP17A1 enzyme.
Abiraterone acetate binds in the active site of the enzyme[29] and coordinates the heme iron through its pyridine nitrogen, mimicking the substrate.