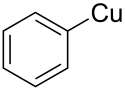
Phenylcopper
Phenylcopper can be obtained by reacting phenyl lithium with copper(I) bromide in diethyl ether.
[3] Phenylcopper is a colorless solid substance that is soluble in pyridine.
Water decomposes phenylcopper to form red copper(I) oxide and varying amounts of benzene and biphenyl.
[4] When dissolved in dimethyl sulfide, phenylcopper forms dimers and trimers (aggregates of two or three molecules).
[5] A diphenylcuprate(I) ion exists that can form a salt with lithium (Li+[Cu(C6H5)2]−),[5] an example of a Gilman reagent.