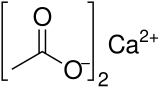
Calcium acetate
Calcium acetate is a chemical compound which is a calcium salt of acetic acid.
An older name is acetate of lime.
Calcium acetate can be prepared by soaking calcium carbonate (found in eggshells, or in common carbonate rocks such as limestone or marble) or hydrated lime in vinegar: Since both reagents would have been available pre-historically, the chemical would have been observable as crystals then.
Pure calcium acetate is yet unknown among minerals.
Calclacite—calcium acetate chloride pentahydrate—is listed as a known mineral,[10] but its genesis is anthropogenic (human-generated, as opposed to naturally occurring).