Polystyrene sulfonate
Common side effects include loss of appetite, gastrointestinal upset, constipation, and low blood calcium.
[1] A polystyrene sulfonate was developed in the 2000s to treat Clostridioides difficile associated diarrhea under the name Tolevamer,[2] but it was never marketed.
[7] Polystyrene sulfonates should not be used in people with obstructive bowel disease and in newborns with reduced gut motility.
[8] A total of 58 cases of intestinal injury including necrosis of the colon have been reported with polystyrene sulfonate as of 2013.
In September 2017, the FDA recommended separating the dosing of polystyrene sulfonate from any other oral medications by at least three hours to avoid any potential interactions.
Recently, the atom transfer radical polymerization (ATRP) of protected styrene sulfonates has been reported,[12][13] leading to well defined linear polymers, as well as more complicated molecular architectures.
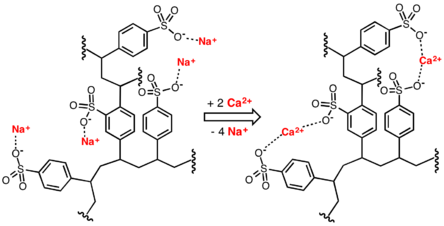
[15] Linear ionic polymers are generally water-soluble, whereas cross-linked materials (called resins) do not dissolve in water.
Sodium polystyrene sulfonate is used as a superplastifier in cement, as a dye improving agent for cotton, and as proton exchange membranes in fuel cell applications.