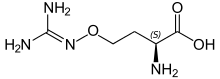
Canavanine
It is structurally related to the proteinogenic α-amino acid L-arginine, the sole difference being the replacement of a methylene bridge (-CH2- unit) in arginine with an oxa group (i.e., an oxygen atom) in canavanine.
Canavanine is accumulated primarily in the seeds of the organisms which produce it, where it serves both as a highly deleterious defensive compound against herbivores (due to cells mistaking it for arginine) and a vital source of nitrogen for the growing embryo.
[7] An example of this ability can be found in the larvae of the tobacco budworm Heliothis virescens, which can tolerate large (lethal concentration 50 or LC50 300 mM) amounts of dietary canavanine.
[9] In contrast, larvae of the tobacco hornworm Manduca sexta can only tolerate tiny amounts (1.0 microgram per kilogram of fresh body weight) of dietary canavanine because their arginine-tRNA ligase has little, if any, discriminatory capacity.
In this insect, the level of radiolabeled L-canavanine incorporated into newly synthesized proteins is barely measurable.