Candida albicans
[11] C. albicans is the most common fungal species isolated from biofilms either formed on (permanent) implanted medical devices or on human tissue.
[14] Nevertheless, these numbers may not truly reflect the true extent of damage this organism causes, given studies indicating that C. albicans can cross the blood–brain barrier in mice.
The oldest reference to thrush, most likely caused by C. albicans, dates back to 400 BC in Hippocrates' work Of the Epidemics describing oral candidiasis.
[2][27] The genome of C. albicans is almost 16Mb for the haploid size (28Mb for the diploid stage) and consists of 8 sets of chromosome pairs called chr1A, chr2A, chr3A, chr4A, chr5A, chr6A, chr7A and chrRA.
[37] However, this different codon usage makes it more difficult to study C. albicans protein-protein interactions in the model organism S. cerevisiae.
In another evolutionary study, introduction of partial CUG identity redefinition (from Candida species) into Saccharomyces cerevisiae clones caused a stress response that negatively affected sexual reproduction.
[44] Spores can form on the pseudohyphae called chlamydospores which survive when put in unfavorable conditions such as dry or hot seasons.
Hyphal cells are invasive and speculated to be important for tissue penetration, colonization of organs and surviving plus escaping macrophages.
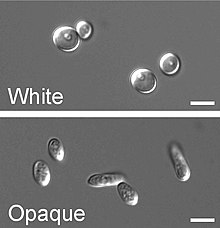
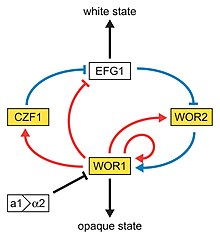
This type of switching does not occur en masse, represents a variability system and it happens independently from environmental conditions.
[62] The discovery of a C. albicans SIR2 implicated in phenotypic switching suggests it, too, has silent regions controlled by SIR2, in which the phenotype-specific genes may reside.
[71][72] Candida is found worldwide but most commonly compromises immunocompromised individuals diagnosed with serious diseases such as HIV and cancer.
[74] These patients predominantly develop oropharyngeal or thrush candidiasis, which can lead to malnutrition and interfere with the absorption of medication.
[77] Healthy people usually do not suffer (severely) from superficial infections caused by a local alteration in cellular immunity as seen by asthma patients that use oral corticosteroids.
[81] Candidiasis is known to cause gastrointestinal (GI) symptoms particularly in immunocompromised patients or those receiving steroids (e.g. to treat asthma) or antibiotics.
Recently, there is an emerging literature that an overgrowth of fungus in the small intestine of non-immunocompromised subjects may cause unexplained GI symptoms.
[9][10][82] Systemic fungal infections (fungemias) including those by C. albicans have emerged as important causes of morbidity and mortality in immunocompromised patients (e.g., AIDS, cancer chemotherapy, organ or bone marrow transplantation).
In turn, C. albicans colonization generates anti-Saccharomyces cerevisiae antibodies (ASCA), increases inflammation, histological scores and pro-inflammatory cytokine expression.
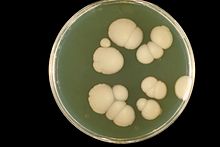
[95] Colonies of white or cream color on fungal culture having a positive germ tube test is strongly indicative of Candida albicans.
[101] Given the fact that candidiasis is the fourth- (to third-) most frequent hospital acquired infection worldwide it leads to immense financial implications.
[103] The immense costs are partly explained by a longer stay in the intensive care unit or hospital in general.
Excessive release of these pro-inflammatory mediators has been shown to exaggerate systemic inflammation leading to vascular injury and damage to vital organs.
In addition, the presence of hyphae and candidalysin are key factors in the activation of GSDMD and the release of Candida from macrophages.
Also using Candida-infected mice, inhibition of GSDMD has been shown to paradoxically improve prognosis and survival, indicating that this protein may be a potential therapeutic target in C. albicans-induced sepsis.
In the maturation step, the biofilm biomass expands, the extracellular matrix accumulates and drug resistance increases.
[117] Due to its nature as a model organism, being an important human pathogen and the alternative codon usage (CUG translated into serine rather than leucine), several specific projects and tools have been created to study C.
[11] The diploid nature and the absence of a sexual cycle make the organism difficult to study, but in the last 20 years, many systems have been developed to observe its genetics.
The URA3 marker (URA3 blaster method) is an often-used strategy in uridine auxotrophic strains; however, studies have shown that differences in URA3 position in the genome can be involved in the pathogeny of C.
[123][124] Due to the aberrant codon usage of C. albicans it is less feasible to use the common host organism (Saccharomyces cerevisiae) for two-hybrid studies.
When proteins interact, the cells will be able to grow on medium lacking histidine due to the activation of the HIS1 reporter gene.
[133][134] C. albicans has been used in combination with carbon nanotubes (CNT) to produce stable electrically conductive bio-nano-composite tissue materials that have been used as temperature-sensing elements.