Carbon dioxide
In the air, carbon dioxide is transparent to visible light but absorbs infrared radiation, acting as a greenhouse gas.
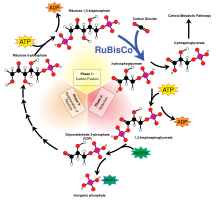
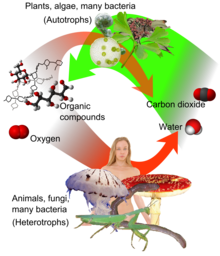
Plants, algae and cyanobacteria use energy from sunlight to synthesize carbohydrates from carbon dioxide and water in a process called photosynthesis, which produces oxygen as a waste product.
Other commercial applications include food and beverage production, metal fabrication, cooling, fire suppression and stimulating plant growth in greenhouses.
[20] In the gas phase, carbon dioxide molecules undergo significant vibrational motions and do not keep a fixed structure.
Only very strong nucleophiles, like the carbanions provided by Grignard reagents and organolithium compounds react with CO2 to give carboxylates: In metal carbon dioxide complexes, CO2 serves as a ligand, which can facilitate the conversion of CO2 to other chemicals.
[26] Photoautotrophs (i.e. plants and cyanobacteria) use the energy contained in sunlight to photosynthesize simple sugars from CO2 absorbed from the air and water: Carbon dioxide is colorless.
[28] This form of glass, called carbonia, is produced by supercooling heated CO2 at extreme pressures (40–48 GPa, or about 400,000 atmospheres) in a diamond anvil.
[31] Phototrophs use the products of their photosynthesis as internal food sources and as raw material for the biosynthesis of more complex organic molecules, such as polysaccharides, nucleic acids, and proteins.
[32] A globally significant species of coccolithophore is Emiliania huxleyi whose calcite scales have formed the basis of many sedimentary rocks such as limestone, where what was previously atmospheric carbon can remain fixed for geological timescales.Plants can grow as much as 50% faster in concentrations of 1,000 ppm CO2 when compared with ambient conditions, though this assumes no change in climate and no limitation on other nutrients.
Additionally, and crucially to life on earth, photosynthesis by phytoplankton consumes dissolved CO2 in the upper ocean and thereby promotes the absorption of CO2 from the atmosphere.
[47] Concentrations of 7% to 10% (70,000 to 100,000 ppm) may cause suffocation, even in the presence of sufficient oxygen, manifesting as dizziness, headache, visual and hearing dysfunction, and unconsciousness within a few minutes to an hour.
[54] At this CO2 concentration, International Space Station crew experienced headaches, lethargy, mental slowness, emotional irritation, and sleep disruption.
[53] However a review of the literature found that a reliable subset of studies on the phenomenon of carbon dioxide induced cognitive impairment to only show a small effect on high-level decision making (for concentrations below 5000 ppm).
Miners, who are particularly vulnerable to gas exposure due to insufficient ventilation, referred to mixtures of carbon dioxide and nitrogen as "blackdamp", "choke damp" or "stythe".
Before more effective technologies were developed, miners would frequently monitor for dangerous levels of blackdamp and other gases in mine shafts by bringing a caged canary with them as they worked.
[67] CO2 is the parameter that changes the fastest (with hygrometry and oxygen levels when humans or animals are gathered in a closed or poorly ventilated room).
It is also why flight attendants instruct passengers, in case of loss of cabin pressure, to apply the oxygen mask to themselves first before helping others; otherwise, one risks losing consciousness.
As an example, the chemical reaction between methane and oxygen: Iron is reduced from its oxides with coke in a blast furnace, producing pig iron and carbon dioxide:[104] Carbon dioxide is a byproduct of the industrial production of hydrogen by steam reforming and the water gas shift reaction in ammonia production.
[105] It is produced by thermal decomposition of limestone, CaCO3 by heating (calcining) at about 850 °C (1,560 °F), in the manufacture of quicklime (calcium oxide, CaO), a compound that has many industrial uses: Acids liberate CO2 from most metal carbonates.
[18]: 3 Other commercial applications include food and beverage production, metal fabrication, cooling, fire suppression and stimulating plant growth in greenhouses.
With the exception of British real ale, draught beer is usually transferred from kegs in a cold room or cellar to dispensing taps on the bar using pressurized carbon dioxide, sometimes mixed with nitrogen.
Aluminium capsules of CO2 are also sold as supplies of compressed gas for air guns, paintball markers/guns, inflating bicycle tires, and for making carbonated water.
[132] Carbon dioxide has also been widely used as an extinguishing agent in fixed fire-protection systems for local application of specific hazards and total flooding of a protected space.
[133] International Maritime Organization standards recognize carbon dioxide systems for fire protection of ship holds and engine rooms.
[135] Carbon dioxide has attracted attention in the pharmaceutical and other chemical processing industries as a less toxic alternative to more traditional solvents such as organochlorides.
Due to the need to operate at pressures of up to 130 bars (1,900 psi; 13,000 kPa), CO2 systems require highly mechanically resistant reservoirs and components that have already been developed for mass production in many sectors.
Its environmental advantages (GWP of 1, non-ozone depleting, non-toxic, non-flammable) could make it the future working fluid to replace current HFCs in cars, supermarkets, and heat pump water heaters, among others.
Methods to administer CO2 include placing animals directly into a closed, prefilled chamber containing CO2, or exposure to a gradually increasing concentration of CO2.
The American Veterinary Medical Association's 2020 guidelines for carbon dioxide induction state that a displacement rate of 30–70% of the chamber or cage volume per minute is optimal for the humane euthanasia of small rodents.
His interpretation was that the rest of the charcoal had been transmuted into an invisible substance he termed a "gas" (from Greek "chaos") or "wild spirit" (spiritus sylvestris).