Carbon nanotube chemistry
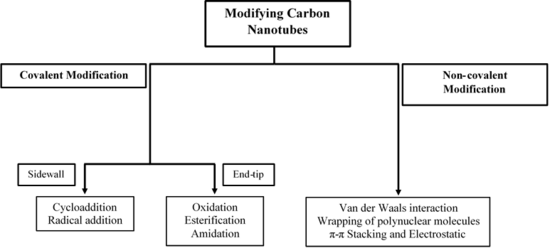
The surface of CNTs can be modified to reduce the hydrophobicity and improve interfacial adhesion to a bulk polymer through chemical attachment.
Although covalent modifications are very stable, the bonding process disrupts the sp2 hybridization of the carbon atoms because a σ-bond is formed.
[11] During acid oxidation, the carbon-carbon bonded network of the graphitic layers is broken allowing the introduction of oxygen units in the form of carboxyl, phenolic and lactone groups,[12] which have been extensively exploited for further chemical functionalisation.
[16] Xing et al. revealed sonication assisted oxidation, with sulfuric and nitric acid, of carbon nanotubes and produced carbonyl and carboxyl groups.
[17] After the oxidation reaction in acidic solution, treatment with hydrogen peroxide limited the damage on the carbon nanotube network.
After expulsion of nitrogen, it forms a covalent bond as an aryl radical:[19][20] Carboxylic groups are used as the precursor for most esterification and amidation reactions.
Carbon nanotubes modified with acyl chloride react readily with highly branched molecules such as poly(amidoamine), which acts as a template for silver ion and later being reduced by formaldehyde.
The most well-known 1,3 cycloaddition reaction involves azomethine ylides reacting with carbon nanotubes, which are of great interest.
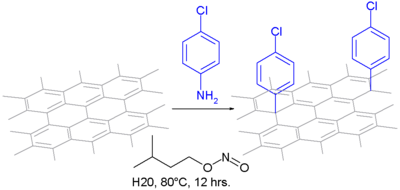
[34] Price et al. demonstrated that stirring carbon nanotubes in water and treating with anilines and oxidizing agents proved to be a milder reaction.
[38] Hirsch was also able to show the nucleophilic addition of amines by generating lithium amides, leading to amino-modified carbon nanotubes.
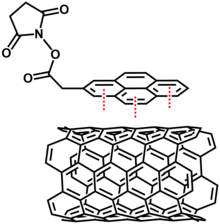
[44] Non-covalent modifications utilize van der Waals forces and π-π interactions by adsorption of polynuclear aromatic compounds, surfactants, polymers or biomolecules.
[6] Some common polynuclear aromatic compounds that are functionalized with hydrophilic or hydrophobic moieties are used to solubilize carbon nanotubes into organic or aqueous solvents.
[6] Proteins have high affinity to carbon nanotubes due to their diversity of amino acids being hydrophobic or hydrophilic.
Other tools are UV spectroscopy where pristine nanotubes show distinct Van Hove singularities where functionalized tubes do not, and simple TGA analysis.