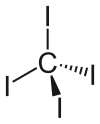
Carbon tetraiodide
[2] The molecule is slightly crowded with short contacts between iodine atoms of 3.459 ± 0.03 Å, and possibly for this reason, it is thermally and photochemically unstable.
[3] It has zero dipole moment due to its symmetrically substituted tetrahedral geometry.
Its synthesis entails AlCl3-catalyzed halide exchange, which is conducted at room temperature:[4] The product crystallizes from the reaction solution.
In an Appel reaction, carbon tetrachloride is used to generate alkyl chlorides from alcohols.
In general, perhalogenated organic compounds should be considered toxic, with the narrow exception of small perfluoroalkanes (essentially inert due to the strength of the C-F bond).