Carbonate
Carbonates are widely used in industry, such as in iron smelting, as a raw material for Portland cement and lime manufacture, in the composition of ceramic glazes, and more.
New applications of alkali metal carbonates include: thermal energy storage,[3][4] catalysis[5] and electrolyte both in fuel cell technology[6] as well as in electrosynthesis of H2O2 in aqueous media.
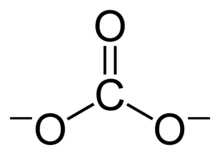
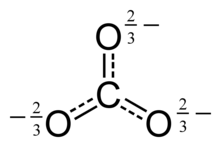
This structure is incompatible with the observed symmetry of the ion, which implies that the three bonds are the same length and that the three oxygen atoms are equivalent.
As in the case of the isoelectronic nitrate ion, the symmetry can be achieved by a resonance among three structures: This resonance can be summarized by a model with fractional bonds and delocalized charges: Metal carbonates generally decompose on heating, liberating carbon dioxide leaving behind an oxide of the metal.
[2] This process is called calcination, after calx, the Latin name of quicklime or calcium oxide, CaO, which is obtained by roasting limestone in a lime kiln: As illustrated by its affinity for Ca2+, carbonate is a ligand for many metal cations.
Of the insoluble metal carbonates, CaCO3 is important because, in the form of scale, it accumulates in and impedes flow through pipes.
Three reversible reactions control the pH balance of blood and act as a buffer to stabilise it in the range 7.37–7.43:[9][10] Exhaled CO2(g) depletes CO2(aq), which in turn consumes H2CO3, causing the equilibrium of the first reaction to try to restore the level of carbonic acid by reacting bicarbonate with a hydrogen ion, an example of Le Châtelier's principle.
The amount of CO2−3 available is on a geological scale and substantial quantities may eventually be redissolved into the sea and released to the atmosphere, increasing CO2 levels even more.
Recent observations of the planetary nebula NGC 6302 show evidence for carbonates in space,[13] where aqueous alteration similar to that on Earth is unlikely.