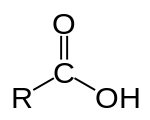
Carboxylic acid
Alternately, it can be named as a "carboxy" or "carboxylic acid" substituent on another parent structure, such as 2-carboxyfuran.
Carboxylic acids usually exist as dimers in nonpolar media due to their tendency to "self-associate".
These longer chain acids tend to be soluble in less-polar solvents such as ethers and alcohols.
Carboxylic acids tend to have higher boiling points than water, because of their greater surface areas and their tendency to form stabilized dimers through hydrogen bonds.
[3][6] By 1H NMR spectrometry, the hydroxyl hydrogen appears in the 10–13 ppm region, although it is often either broadened or not observed owing to exchange with traces of water.
In general, industrial routes to carboxylic acids differ from those used on a smaller scale because they require specialized equipment.
Preparative methods for small scale reactions for research or for production of fine chemicals often employ expensive consumable reagents.
Many reactions produce carboxylic acids but are used only in specific cases or are mainly of academic interest.
The conversion of amino acids into peptides is a significant biochemical process that requires ATP.
The condensation produces water, however, which can hydrolyze the anhydride back to the starting carboxylic acids.
Under acid-catalyzed conditions, carboxylic acids will react with alcohols to form esters via the Fischer esterification reaction, which is also an equilibrium process.
The Vilsmaier reagent (N,N-Dimethyl(chloromethylene)ammonium chloride; [ClHC=N+(CH3)2]Cl−) is a highly chemoselective agent for carboxylic acid reduction.
It selectively activates the carboxylic acid to give the carboxymethyleneammonium salt, which can be reduced by a mild reductant like lithium tris(t-butoxy)aluminum hydride to afford an aldehyde in a one pot procedure.
The resulting oxonium ion 2 is activated towards nucleophilic attack and has a good leaving group, setting it apart from a normal carboxylic acid.
PCl5 reacts with carboxylic acids in a 1:1 ratio, and produces phosphorus(V) oxychloride (POCl3) and hydrogen chloride (HCl) as byproducts.
A second equivalent will attack the carbonyl group to create a geminal alkoxide dianion, which is protonated upon workup to give the hydrate of a ketone.
[12] The acid dissociation constant of •COOH has been measured using electron paramagnetic resonance spectroscopy.