Celecoxib
[20] It has been used to reduce colon and rectal polyps in people with familial adenomatous polyposis, but it is not known if it decreases rates of cancer,[7] so it is not a good choice for this reason.
Moderate to severe liver impairment or GI toxicity can occur with or without warning symptoms in people treated with NSAIDs.
A 2013 meta-analysis of hundreds of clinical trials found that coxibs (the class of drugs that includes celecoxib) increase the risk of major cardiovascular problems by about 37% over placebo.
"[9] In 2005, a study published in the Annals of Internal Medicine found that cardiovascular effects of COX-2 inhibitors differ, depending on the drug.
[27] In a 2006 meta-analysis of randomized control studies, the cerebrovascular events associated with COX-2 inhibitors were examined, but no significant risks were found when compared to nonselective NSAIDs or placebos.
[5] The risk of bleeding and gastric ulcers also increases further when selective serotonin reuptake inhibitors (SSRI) are used in combination with celecoxib.
[5] A highly selective reversible inhibitor of the COX-2 isoform of cyclooxygenase, celecoxib inhibits the transformation of arachidonic acid to prostaglandin precursors.
[5] COX-1 is traditionally defined as a constitutively expressed "housekeeping" enzyme and plays a role in the protection of the gastrointestinal mucosa, kidney hemodynamics, and platelet thrombogenesis.
[30][31] COX-2, on the contrary, is extensively expressed in cells involved in inflammation and is upregulated by bacterial lipopolysaccharides, cytokines, growth factors, and tumor promoters.
[34] In theory, this selectivity allows celecoxib and other COX-2 inhibitors to reduce inflammation (and pain) while minimizing gastrointestinal adverse drug reactions (e.g. stomach ulcers) that are common with nonselective NSAIDs.
[35] For its use in reducing colon polyps, celecoxib affects genes and pathways involved in inflammation and malignant transformation in tumors, but not normal tissues.
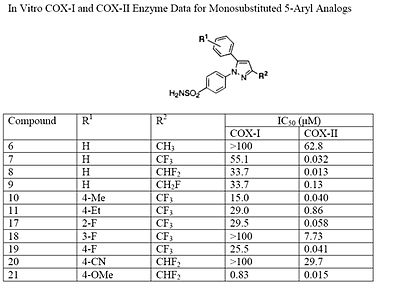
At the 3-position of the pyrazole, a trifluoromethyl or difluoromethyl provides superior selectivity and potency compared to a fluoromethyl or methyl substitution (see structures 6 – 9).
[37] To explain the selectivity of celecoxib, it is necessary to analyze the free energy of binding difference between the drug molecule and COX-1 compared to COX-2 enzymes.
[38] This mutation appears to contribute to COX-2 selectivity by creating steric hindrance between the sulfonamide oxygen and the methyl group of Ile523 that effectively destabilizes the celecoxib-COX-1 complex.
Celecoxib and other COX-2 selective inhibitors, valdecoxib, parecoxib, and mavacoxib, were discovered by a team at the Searle division of Monsanto led by John Talley.
The court ruled in favor of Searle in 2004, holding in essence that the university had claimed a method requiring, yet provided no written description of, a compound that could inhibit COX-2, and therefore the patent was invalid.
The court ruled in favor of Searle, holding in essence that the university had claimed a method requiring, yet provided no written description of, a compound that could inhibit COX-2, and therefore the patent was invalid.
[53] Pfizer responded to Public Citizen's concerns with assurances that they are truthfully advertising the risk and benefits of Celebrex as set forth by the FDA.
[51] In 2001, the US Food and Drug Administration (FDA) released the full results of the Pfizer and Pharmacia study which showed that they had withheld crucial data.
[51] By 2012, a federal judge unsealed "thousands of pages of internal documents and depositions" in a "long-running securities fraud case against Pfizer.
"[51] In March 2009, Scott S. Reuben, former chief of acute pain at Baystate Medical Center, Springfield, Massachusetts, revealed that the data for 21 studies he had authored for the efficacy of the drug (along with others such as Vioxx) had been fabricated.
When we decided to support Dr. Reuben's research, he worked for a credible academic medical center and appeared to be a reputable investigator",[55][56] the documents unsealed in 2012, revealed that by February 2000, Pharmacia employees had devised a strategy to present the findings.
[64][65][66][67][68] A meta-analysis considering trials of celecoxib as an adjunctive treatment in bipolar disorder was inconclusive citing low evidence quality.
[64] It has been used to reduce colon and rectal polyps in people with familial adenomatous polyposis, but it is not known if it decreases rates of cancer,[7] so it is not a good choice for this reason.
[69] Small-scale clinical trials in very high-risk people (belonging to FAP families) showed celecoxib can prevent polyp growth.
The discovery of these additional targets has generated much controversy, and the initial assumption that celecoxib reduces tumor growth primarily by the inhibition of COX-2 became contentious.
[76] Karen Seibert and colleagues have published research showing antiangiogenic and antitumor activity of celecoxib in animal models.