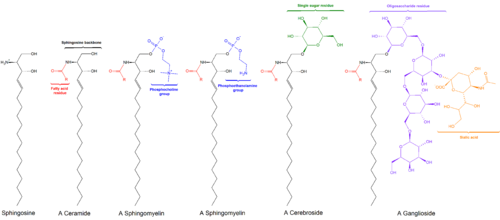
Cerebroside
Cerebrosides (monoglycosylceramides) are a group of glycosphingolipids which are important components of animal muscle and nerve cell membranes.
The biosynthesis of monoglycosylceramides requires a direct transfer of the carbohydrate moiety from a sugar-nucleotide, such as uridine 5-diphosphate(UDP)-galactose, or UDP-glucose to the ceramide unit.
Synthesis of galactosylceramide, and glucosylceramide occurs on the lumenal surface of the endoplasmic reticulum, and on the cytosolic side of the early Golgi membranes respectively.
The melting point of cerebrosides is considerably greater than physiological body temperature, >37.0 °C, giving glycolipids a paracrystalline, similar to liquid crystal structure.
Monoglycosylceramides in conjunction with cholesterol are prevalent in the lipid-raft micro domain, which are important sites in the binding of proteins, and enzyme-receptor interactions.
Reversed-phase HPLC is now the standard method for separation of molecular species, often after benzoylation, enabling lipids to be detected by UV spectrophotometry.
Clinical features include acroparaesthesia (tingling, pins and needles sensation in the extremities)[3] b) Galactocerebroside (galactosylceramidase) accumulation – Krabbe disease.