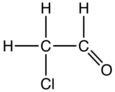
Chloroacetaldehyde
Like some related compounds, it is highly electrophilic reagent and a potentially dangerous alkylating agent.
[6] Water free chloroacetaldehyde is prepared from the hydrate by azeotropic distillation with chloroform, toluene, or carbon tetrachloride.
Relevant to its occurrence in humans, it arises via the isomerization of chloroethylene oxide, a metabolite of vinyl chloride.
[5] Chloroacetaldehyde is a building block in the synthesis of the pharmaceuticals altizide, polythiazide, brotizolam, and ciclotizolam.
[1] Based on data collected from human studies in 1962, exposures to 45 ppm of chloroacetaldehyde were found to be disagreeable and caused conjunctival irritation to the subjects.