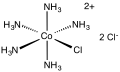
Chloropentamminecobalt chloride
The salt is prepared with a two-step process starting with oxidizing a solution of cobalt chloride and ammonia.
[1][2] This intermediate is then heated to induce coordination of one of the outer sphere chloride ligands: The dication [Co(NH3)5Cl]2+ has idealized C4v symmetry.
With concentrated sulfuric acid, chloropentaamminecobalt(III) chloride forms the hydrogen sulfate complex [Co(NH3)5OSO3H]2+.
Cobalt complexes have been of long-standing interest in inorganic chemistry because they are numerous, easily prepared, and colorful.
Also known as CPACC the molecule is investigated in relation with limiting the magnesium available for mitochondria and subsequent metabolic health benefits.