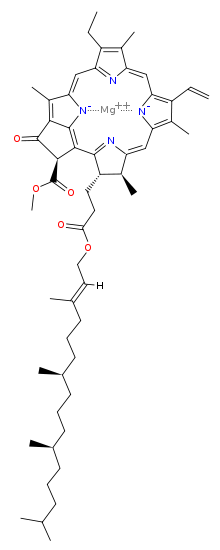
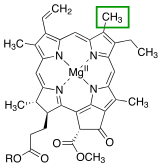
Chlorophyll a
[6] Chlorophyll a is essential for most photosynthetic organisms to release chemical energy but is not the only pigment that can be used for photosynthesis.
[5] Chlorophyll a can also be found in very small quantities in the green sulfur bacteria, an anaerobic photoautotroph.
[8] The porphyrin ring of bacteriochlorophyll is saturated, and lacking alternation of double and single bonds causing variation in absorption of light.
Different side chains characterize each type of chlorophyll molecule, and alters the absorption spectrum of light.
[5] Once detached from the porphyrin ring, phytol becomes the precursor of two biomarkers, pristane and phytane, which are important in the study of geochemistry and the determination of petroleum sources.
[13] In most plants, chlorophyll is derived from glutamate and is synthesised along a branched pathway that is shared with heme and siroheme.
Accessory photosynthetic pigments broaden the spectrum of light absorbed, increasing the range of wavelengths that can be used in photosynthesis.
Light energy radiating onto the chloroplast strikes the pigments in the thylakoid membrane and excites their electrons.
Phytoplankton can be affected indirectly by climatic factors, such as changes in water temperatures and surface winds.