Chromium(III) sulfide
Chromium(III) sulfide is the inorganic compound with the formula Cr2S3.
Chromium sulfides are usually nonstoichiometric compounds, with formulas ranging from CrS to Cr0.67S (corresponding to Cr2S3).
Chromium(III) sulfide can be prepared through the reaction of a stoichiometric mixture of the elements at 1000 °C[2] It is a solid that is insoluble in water.
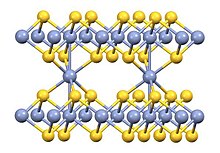
According to X-ray crystallography, its structure is a combination of that of nickel arsenide (1:1 stoichiometry) and Cd(OH)2 (1:2 stoichiometry).
Some metal-metal bonding is indicated by the short Cr-Cr distance of 2.78 Å.