Chromic acid
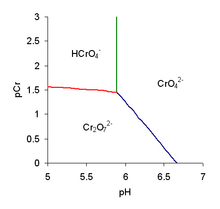
In this tetrahedral oxyanion, three Cr-O bond lengths are 156 pm and the Cr-OH bond is 201 pm[5] [HCrO4]− condenses to form dichromate: Furthermore, the dichromate can be protonated: Loss of the second proton occurs in the pH range 4–8, making the ion [HCrO4]− a weak acid.
[citation needed] Molecular chromic acid could in principle be made by adding chromium trioxide to water (cf.
Under these conditions deep red crystals of chromium trioxide precipitate from the mixture, without further colour change.
Chromic acid is an intermediate in chromium plating, and is also used in ceramic glazes, and colored glass.
[8] Furthermore, the acid leaves trace amounts of paramagnetic chromic ions (Cr3+) that can interfere with certain applications, such as NMR spectroscopy.
Chromic acid was widely used in the musical instrument repair industry, due to its ability to "brighten" raw brass.
[11] Chromic acid is capable of oxidizing many kinds of organic compounds and many variations on this reagent have been developed: In organic chemistry, dilute solutions of chromic acid can be used to oxidize primary or secondary alcohols to the corresponding aldehydes and ketones.
Because the oxidation is signaled by a color change from orange to brownish green (indicating chromium being reduced from oxidation state +6 to +3), chromic acid is commonly used as a lab reagent in high school or undergraduate college chemistry as a qualitative analytical test for the presence of primary or secondary alcohols, or aldehydes.
Chromium trioxide and chromic acids are strong oxidizers and may react violently if mixed with easily oxidizable organic substances.