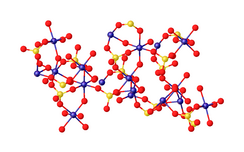
Chromium(III) sulfate
[3] Chromium(III) sulfate are commonly obtained from the wastes of chromate oxidations of various organic compounds.
Anthraquinone and quinone are produced on large scale by the x treatment of respectively anthracene and phenol with chromic acid.
Extraction of chromite ore with sulfuric acid in the presence of some chromate gives solutions of chromium(III) sulfate contaminated with other metal ions.
[4] Basic chromium sulfate is produced from chromate salts by reduction with sulfur dioxide, although other methods exist.
[4][5] The reduction could formally be written: Since 33% of the anion charges are due to hydroxy ions the basicity is 33% (but in tanning jargon it is known as 33% reduced).