Chronic myelogenous leukemia
CML is largely treated with targeted drugs called tyrosine-kinase inhibitors (TKIs) which have led to dramatically improved long-term survival rates since 2001.
In these cases, it may be diagnosed incidentally with an elevated white blood cell count on a routine laboratory test.
[5] Exposure to ionising radiation appears to be a risk factor, based on a 50 fold higher incidence of CML in Hiroshima and Nagasaki nuclear bombing survivors.
Moreover, the BCR-ABL protein inhibits DNA repair, causing genomic instability and making the cell more susceptible to developing further genetic abnormalities.
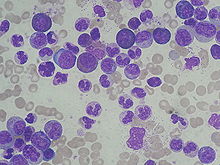
[8] CML is often suspected on the basis of a complete blood count, which shows increased granulocytes of all types, typically including mature myeloid cells.
[10] Precise patient staging based on clinical markers and personal genomic profile will likely prove beneficial in the assessment of disease history with respect to progression risk.
[12] Blast crisis is the final phase in the evolution of CML, and behaves like an acute leukemia, with rapid progression and short survival.
[20] In the past, antimetabolites (e.g., cytarabine, hydroxyurea), alkylating agents, interferon alfa 2b, and steroids were used as treatments of CML in the chronic phase, but since the 2000s have been replaced by Bcr-Abl tyrosine-kinase inhibitors[21] drugs that specifically target BCR-ABL, the constitutively activated tyrosine kinase fusion protein caused by the Philadelphia chromosome translocation.
Despite the move to replacing cytotoxic antineoplastics (standard anticancer drugs) with tyrosine kinase inhibitors sometimes hydroxyurea is still used to counteract the high leukocyte counts encountered during treatment with tyrosine kinase inhibitors like imatinib; in these situations it may be the preferred myelosuppressive agent due to its relative lack of leukemogenic effects and hence the relative lack of potential for secondary haematologic malignancies to result from treatment.
[22] IRIS, an international study that compared interferon/cytarabine combination and the first of these new drugs imatinib, with long-term follow up, demonstrated the clear superiority of tyrosine-kinase-targeted inhibition over existing treatments.
Since the advent of imatinib, CML has become the first cancer in which a standard medical treatment may give to the patient a normal life expectancy.
In 2010, nilotinib and dasatinib were also approved for first-line therapy, making three drugs in this class available for treatment of newly diagnosed CML.
In 2012, radotinib joined the class of novel agents in the inhibition of the BCR-ABL protein and was approved in South Korea for people resistant to or intolerant of imatinib.
Bosutinib received US FDA and EU European Medicines Agency approval on 4 September 2012, and 27 March 2013, respectively for the treatment of adults with Philadelphia chromosome-positive (Ph+) chronic myelogenous leukemia (CML) with resistance, or intolerance to prior therapy.
[citation needed] Two approaches were developed to the treatment of CML as a result: In 2007, Chemgenex released results of an open-label Phase 2/3 study (CGX-635-CML-202) that investigated the use of a non BCR-ABL targeted agent omacetaxine, administered subcutaneously (under the skin) in patients who had failed with imatinib and exhibited T315I kinase domain mutation.
Just as with second-generation TK inhibitors, early approval is being sought to extend the use of ponatinib to newly diagnosed CML also.
[citation needed] In October 2021, the Food and Drug Administration approved asciminib (Scemblix), the first TK inhibitor specifically targeting the ABL1 myristoyl pocket (STAMP) via allosteric binding, as a third-line option for patients with chronic-phase-CML.
[31] In 2005, encouraging but mixed results of vaccination were reported with the BCR/ABL1 p210 fusion protein in patients with stable disease, with GM-CSF as an adjuvant.
[34] The American Cancer Society estimates that in 2014, about 5,980 new cases of chronic myeloid leukemia were diagnosed, and about 810 people died of the disease.