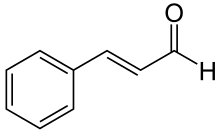
Cinnamaldehyde
[2] This pale yellow, viscous liquid occurs in the bark of cinnamon trees and other species of the genus Cinnamomum.
[3] Cinnamaldehyde was isolated from cinnamon essential oil in 1834 by Jean-Baptiste Dumas and Eugène-Melchior Péligot[4] and synthesized in the laboratory by the Italian chemist Luigi Chiozza in 1854.
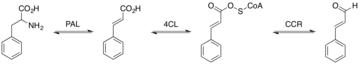
[7] Deamination of L-phenylalanine into cinnamic acid is catalyzed by phenylalanine ammonia lyase (PAL).
The most obvious application for cinnamaldehyde is as flavoring in chewing gum, ice cream, candy, e-liquid and beverages; use levels range from 9 to 4,900 parts per million (ppm) (that is, less than 0.5%).
Almond, apricot, butterscotch, and other aromas may partially employ the compound for their pleasant smells.
[18] A concentration of 29 ppm of cinnamaldehyde kills half of Aedes aegypti mosquito larvae in 24 hours.
Dihydrocinnamyl alcohol (3-phenylpropanol) occurs naturally but is produced by double hydrogenation of cinnamaldehyde.
Cinnamyl alcohol similarly occurs naturally and has the odor of lilac but can be also produced starting from cinnamaldehyde.
[25] Cinnamaldehyde may cause allergic contact stomatitis in sensitised individuals, however allergy to the compound is believed to be uncommon.
Cinnamaldehyde is a dietary antimutagen that effectively inhibits both induced and spontaneous mutations.