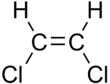
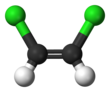
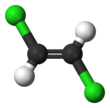
1,2-Dichloroethylene
The two compounds are isomers, each being colorless liquids with a sweet odor.
[4] cis-DCE, the Z isomer, is obtainable by the controlled chlorination of acetylene: Industrially both isomers arise as byproducts of the production of vinyl chloride, which is produced on a vast scale.
[6] These compounds have "moderate oral toxicity to rats".
[1] The dichloroethylene isomers occur in some polluted waters and soils, as the decomposition products of trichloroethylene.
Significant attention has been paid to their further degradation, e.g. by iron particles.