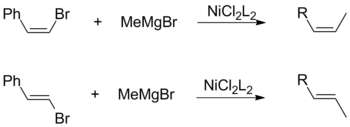Kumada coupling
The procedure uses transition metal catalysts, typically nickel or palladium, to couple a combination of two alkyl, aryl or vinyl groups.
Despite the subsequent development of alternative reactions (Suzuki, Sonogashira, Stille, Hiyama, Negishi), the Kumada coupling continues to be employed in many synthetic applications, including the industrial-scale production of aliskiren, a hypertension medication, and polythiophenes, useful in organic electronic devices.
The first investigations into the catalytic coupling of Grignard reagents with organic halides date back to the 1941 study of cobalt catalysts by Morris S. Kharasch and E. K.
[3] In 1971, Tamura and Kochi elaborated on this work in a series of publications demonstrating the viability of catalysts based on silver,[4] copper[5] and iron.
[6] However, these early approaches produced poor yields due to substantial formation of homocoupling products, where two identical species are coupled.
[7] Subsequently, many additional coupling techniques have been developed, culminating in the 2010 Nobel Prize in Chemistry recognized Ei-ichi Negishi, Akira Suzuki and Richard F. Heck for their contributions to the field.
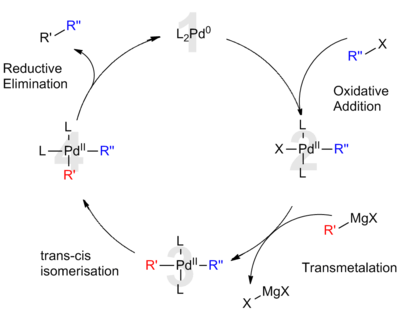
Finally, reductive elimination of (4) forms a carbon–carbon bond and releases the cross coupled product while regenerating the Pd(0) catalyst (1).
Examination by Vicic and coworkers using tridentate terpyridine ligand identified intermediates of a Ni(II)-Ni(I)-Ni(III) catalytic cycle,[10] suggesting a more complicated scheme.
In place of the halide reagent pseudohalides can also be used, and the coupling has been shown to be quite effective using tosylate[12] and triflate[13] species in variety of conditions.
Having no π-electrons, alkyl halides require different oxidative addition mechanisms than aryl or vinyl groups, and these processes are currently poorly understood.
[2] Due to the high reactivity of the Grignard reagent, Kumada couplings have limited functional group tolerance which can be problematic in large syntheses.
[17] Conversely, a Kumada coupling using vinylic Grignard reagents proceeds without stereospecificity to form a mixture of cis- and trans-alkenes.
[23] In 1992, McCollough and Lowe developed the first synthesis of regioregular polyalkylthiophenes by utilizing the Kumada coupling scheme pictured below, which requires subzero temperatures.