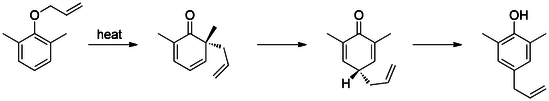
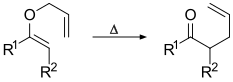Claisen rearrangement
[2][3][4][5] The Claisen rearrangement is an exothermic, concerted (bond cleavage and recombination) pericyclic reaction.
Crossover experiments eliminate the possibility of the rearrangement occurring via an intermolecular reaction mechanism and are consistent with an intramolecular process.
[15][16] The Bellus–Claisen rearrangement is the reaction of allylic ethers, amines, and thioethers with ketenes to give γ,δ-unsaturated esters, amides, and thioesters.
[20][21] The Bellus-Claisen offers synthetic chemists a unique opportunity for ring expansion strategies.
The Eschenmoser–Claisen rearrangement proceeds by heating allylic alcohols in the presence of N,N-dimethylacetamide dimethyl acetal to form a γ,δ-unsaturated amide.
The E- and Z-configured silylketene acetals lead to anti and syn rearranged products, respectively.
[32] However, microwave assisted heating in the presence of KSF-clay or propionic acid have demonstrated dramatic increases in reaction rate and yields.
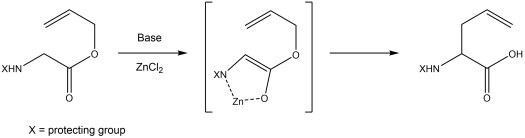
[33][34] Mechanism:[12] The Kazmaier-Claisen rearrangement is the reaction of an unsaturated amino acid ester with a strong base (such as lithium diisopropylamide) and a metal salt at –78 °C to give a chelated enolate as intermediate.
[35][36] While different metal salts can be used to form the enolate, the use of zinc chloride results in the highest yield and gives the best stereospecificity.
[37] The enolate species rearranges at –20 °C to form an amino acid with an allylic side chain in α-position.