Nucleophilic conjugate addition
With α,β-unsaturated carbonyl compounds such as cyclohexenone it can be deduced from resonance structures that the β position is an electrophilic site which can react with a nucleophile.
The negative charge carried by the nucleophile is now delocalized in the alkoxide anion and the α carbon carbanion by resonance.
Conjugate addition is effective in the formation of new carbon-carbon bonds with the help of organometallic reagents such as the organozinc iodide reaction with methylvinylketone.
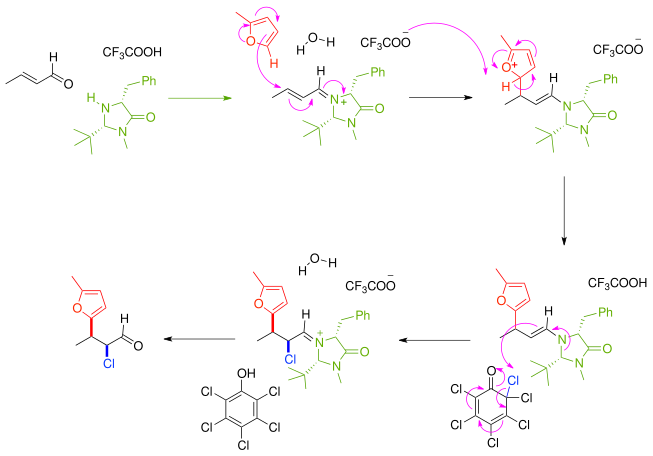
After removal of the amine catalyst the ketone is effectively functionalized with a nucleophile and an electrophile with syn:anti ratio of 8:1 and 97% enantiomeric excess.
[3] This principle is also applied in an enantioselective multicomponent domino conjugated addition of nucleophilic thiols such as benzylmercaptan and electrophilic DEAD.

![(4R',5R')-5-(5-Ethyl-2,2-dimethyl-[1,3]-dioxolan-4-yl)-pentan-2-one](http://upload.wikimedia.org/wikipedia/commons/7/7b/Conjugateadditionexample.png)