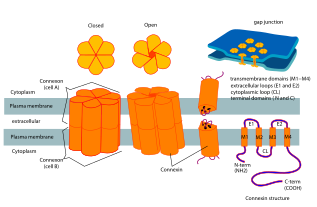
Connexin
Gap junctions are essential for many physiological processes, such as the coordinated depolarization of cardiac muscle, proper embryonic development, and the conducted response in microvasculature.
The connexin gene family is diverse, with twenty-one identified members in the sequenced human genome, and twenty in the mouse (nineteen of which are orthologous pairs).
The various connexins have been observed to combine into both homomeric and heteromeric gap junctions, each of which may exhibit different functional properties including pore conductance, size selectivity, charge selectivity, voltage gating, and chemical gating.
It has been suggested that this short life span allows for more finely regulated physiological processes to take place, such as in the myometrium.
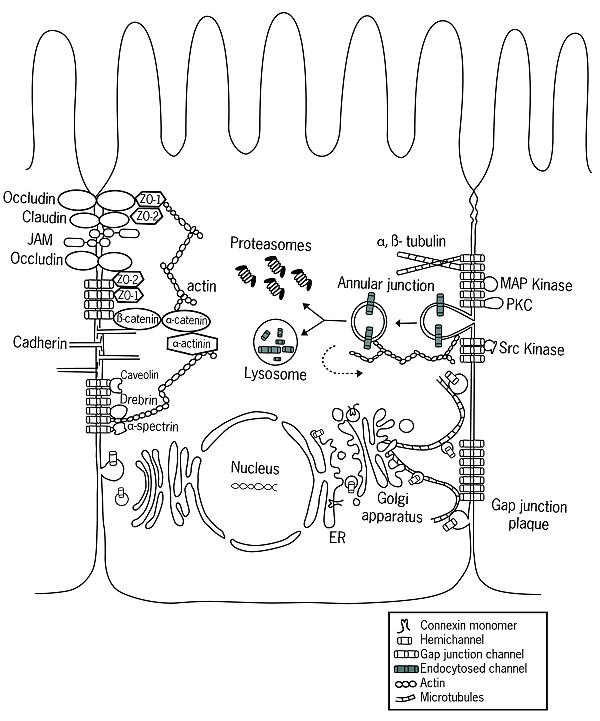
It is also in the ER that the oligomerization of connexin molecules into hemichannels begins, a process which may continue in the UR-Golgi intermediate compartment as well.
Instead, the channels formed by these proteins (called pannexins) act as very large transmembrane pores that connect the intra- and extracellular compartments.
By using specific connexin knockout mice, studies revealed that cell coupling is essential for visual signaling.
[17] Decrock et al.. have discussed a multilevel platform via which connexins and pannexins can influence the following cellular functions within a tissue: (1) connexin gap junctional channels (GJCs) enable direct cell-cell communication of small molecules, (2) connexin hemichannels and pannexin channels can contribute to autocrine/paracrine signaling pathways, and (3) different structural domains of these proteins allow for channel-independent functions, such as cell-cell adhesion, interactions with the cytoskeleton, and the activation of intracellular signaling pathways.
[18] Thus, connexins and pannexins have multifaceted contributions to brain development and specific processes in the neuro-glio-vascular unit, including synaptic transmission and plasticity, glial signaling, vasomotor control, cell movement, and blood-brain barrier integrity in the mature CNS.
This may have functional consequence because the energy status of a cell could be controlled via connexin expression and channel formation.