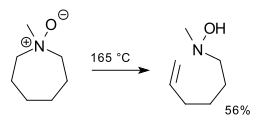
Cope reaction
Illustrative is a synthesis of methylenecyclohexane:[5] The reaction proceeds through the Ei pathway, with an intramolecular, cyclic 5-membered transition state.
)[6][7][8] This organic reaction is closely related to the Hofmann elimination,[2] but the base is a part of the leaving group.
Sulfoxides can undergo an essentially identical reaction to produce sulfenic acids, which is important in the antioxidant chemistry of garlic and other alliums.
The reverse or retro-Cope elimination has been reported, in which an N,N-disubstituted hydroxylamine reacts with an alkene to form a tertiary N-oxide.
[9][10] The reaction is a form of hydroamination and can be extended to the use of unsubstituted hydroxylamine, in which case oximes are produced.