Cope rearrangement
[5] The chair transition state illustrated here is preferred in open-chain systems (as shown by the Doering-Roth experiments).
It is currently generally accepted that most Cope rearrangements follow an allowed concerted route through a Hückel aromatic transition state and that a diradical intermediate is not formed.
However, the concerted reaction can often be asynchronous and electronically perturbed systems may have considerable diradical character at the transition state.
In asymmetric dienes one often needs to consider the stereochemistry, which in the case of pericyclic reactions, such as the Cope rearrangement, can be predicted with the Woodward–Hoffmann rules and consideration of the preference for the chair transition state geometry.
[8][9] In the oxy-Cope rearrangement, a hydroxyl group is added at C3 forming an enal or enone after keto-enol tautomerism of the intermediate enol.
Consequently, the anion-accelerated oxy-Cope reaction can proceed with high efficiency even in systems that do not permit efficient orbital overlap, as illustrated by a key step in the synthesis periplanone B:[15] The corresponding neutral oxy-Cope and siloxy-Cope rearrangements failed, giving only elimination products at 200 °C.
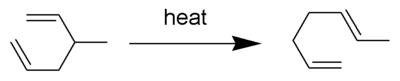



![Example from [Stuart Schreiber]](http://upload.wikimedia.org/wikipedia/commons/thumb/5/5a/Schreiber.png/600px-Schreiber.png)