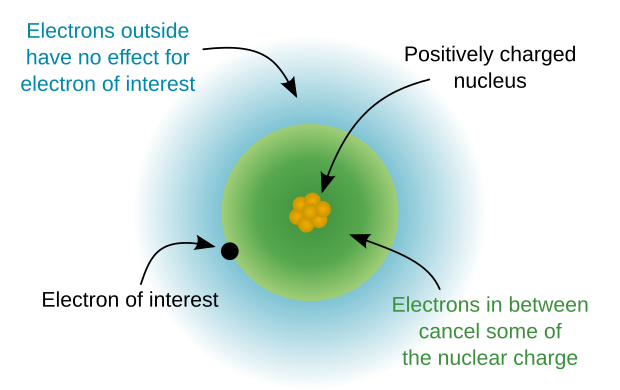
Core electron
A more complex explanation of the difference between core and valence electrons can be described with atomic orbital theory.
The increase in energy for subshells of increasing angular momentum in larger atoms is due to electron–electron interaction effects, and it is specifically related to the ability of low angular momentum electrons to penetrate more effectively toward the nucleus, where they are subject to less screening from the charge of intervening electrons.
For heavy atoms, the core radius grows slightly with increasing number of electrons.
When ionized by flame or ultraviolet radiation, atomic cores, as a rule, also remain intact.
[4] Since the core charge increases as you move across a row of the periodic table, the outer-shell electrons are pulled more and more strongly towards the nucleus and the atomic radius decreases.
This can be used to explain a number of periodic trends such as atomic radius, first ionization energy (IE), electronegativity, and oxidizing.
As a core charge increases, the valence electrons are more strongly attracted to the nucleus, and the atomic radius decreases across the period.
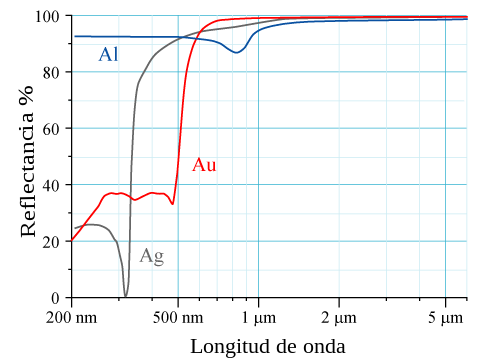
Physical properties affected by these relativistic effects include lowered melting temperature of mercury and the observed golden colour of gold and caesium due to narrowing of energy gap.
This will either excite the electron to an empty valence shell or cause it to be emitted as a photoelectron due to the photoelectric effect.
The resulting atom will have an empty space in the core electron shell, often referred to as a core-hole.