Corey–Itsuno reduction
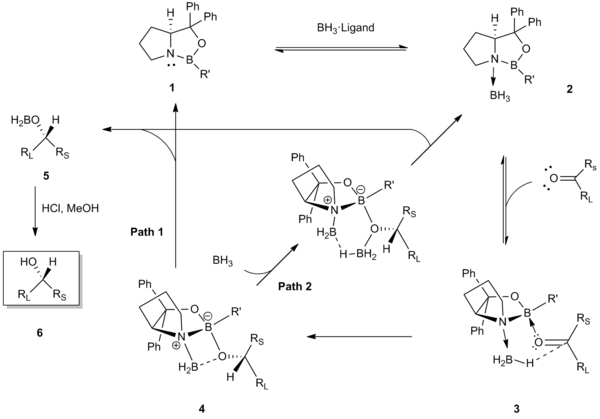
[1] Several years later in 1987, E. J. Corey and coworkers developed the reaction between chiral amino alcohols and borane (BH3), generating oxazaborolidine products which were shown to rapidly catalyze the enantioselective reduction of achiral ketones in the presence of BH3•THF.
This preferential binding in 3 acts to minimize 1,3-allylic strain between the ketone (the large RL substituent directed away) and the R’ group of the catalyst, and aligns the carbonyl and the coordinated borane for a favorable, face-selective hydride transfer through a six-membered transition state 4.
[5] The CBS reduction has proven to be an effective and powerful method to reduce a wide range of different types of ketones in both a stereoselective and chemoselective manner.
Substrates include a large variety of aryl-aliphatic, di-aliphatic, di-aryl, α,β unsaturated enone and ynone systems, as well as ketones containing heteroatoms.
The presence of water in the reaction mixture has been shown to have a significant effect on enantiomeric excesses, and thus the CBS reduction must be conducted under anhydrous conditions.
Commercially available solutions of BH3•THF evaluated by Nettles et al. were shown to contain trace amounts of borohydride species, which participate in nonselective reductions that led to the diminished enantioselectivity.
Jones et al. utilized the CBS reduction in the total synthesis of MK-0417, a water-soluble carbonic anhydrase inhibitor which has been used therapeutically to reduce intraocular pressure.
Asymmetric reduction of a 1,1,1-trichloro-2-keto compound is the first stage of the Corey–Link reaction for synthesis of amino acids and related structures with a choice of either natural or un-natural stereochemistry and various side-chains.