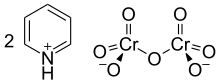
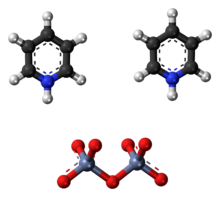
Cornforth reagent
In its chemical structure and functions it is closely related to other compounds made from hexavalent chromium oxide, such as pyridinium chlorochromate and Collins reagent.
The powder is stable in air, not particularly hygroscopic and has an almost neutral pH that facilitates its handling; it is only slightly acidic owing to the presence of pyridinium cations.
They mentioned that reaction of saturated primary alcohols with PDC, using dimethylformamide as solvent, results in oxidation to carboxylic acids rather than aldehydes.
The slow oxidation rate for some alcohols can be accelerated by the addition of molecular sieves, organic acids or acetic anhydride or of their combinations.
The acceleration by molecular sieves works best when their pore diameter is about 0.3 nm, and it is apparently unrelated to their water absorption capability.
Reaction acceleration depends not only on the additives but also on their form, so all reagents are preferred dry and freshly prepared, and PDC and molecular sieves should be finely ground.