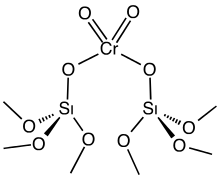
Phillips catalyst
A heterogeneous catalyst, it consists of a chromium oxide supported on silica gel.
[1] Polyethylene, the most-produced synthetic polymer, is produced industrially by the polymerization of ethylene: Although exergonic (i.e., thermodynamically favorable), the reaction requires catalysts.
The solid precatalyst is then calcined in air to give the active catalyst.
The active catalyst is often depicted as a chromate ester bound to the silica surface.
The mechanism for the polymerization process is the subject of much research, the central question being the structure of the active species, which is assumed to be an organochromium compound.