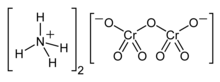
Ammonium dichromate
[5] It is also used as a mordant for dyeing pigments, in manufacturing of alizarin, chrome alum, leather tanning and oil purification.
[6] The volcano demonstration involves igniting a pile of the salt, which initiates the following exothermic conversion:- [8] Like ammonium nitrate, it is thermodynamically unstable.
[9][10] Its decomposition reaction proceeds to completion once initiated, producing voluminous dark green powdered chromium(III) oxide.
The characteristic darkening of (NH4)2Cr2O7 crystals as a consequence of the onset of decomposition can be ascribed to the dissociative loss of ammonia accompanied by progressive anion condensation to Cr3O2−10, Cr4O2−13, etc., ultimately yielding CrO3.
Ammonium dichromate, in the presence of Mg(HSO4)2 and wet SiO2 can act as a very efficient reagent for the oxidative coupling of thiols under solvent free conditions.
[9] In 1986, two workers were killed and 14 others injured at Diamond Shamrock Chemicals in Ashtabula, Ohio, when 2,000 lb (910 kg) of ammonium dichromate exploded as it was being dried in a heater.