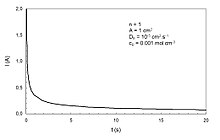
Cottrell equation
In electrochemistry, the Cottrell equation describes the change in electric current with respect to time in a controlled potential experiment, such as chronoamperometry.
Specifically it describes the current response when the potential is a step function in time.
[1] For a simple redox event, such as the ferrocene/ferrocenium couple, the current measured depends on the rate at which the analyte diffuses to the electrode.
The Cottrell equation describes the case for an electrode that is planar but can also be derived for spherical, cylindrical, and rectangular geometries by using the corresponding Laplace operator and boundary conditions in conjunction with Fick's second law of diffusion.
Deviations from linearity can be expected at very short time scales due to non-ideality in the potential step.