Voltammetry
Voltammetric methods involve electrochemical cells, and investigate the reactions occurring at electrode/electrolyte interfaces.
It is considered a dynamic electrochemical method as the applied potential is varied over time and the corresponding changes in current are measured.
[4] Most experiments control the potential (volts) of an electrode in contact with the analyte while measuring the resulting current (amperes).
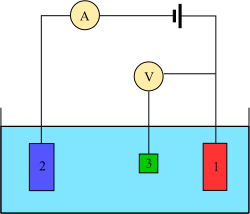
The cell consists of an analyte solution, an ionic electrolyte, and two or three electrodes, with oxidation and reduction reactions occurring at the electrode/electrolyte interfaces.
The shape of the curves depends on the speed of potential variation, (nature of driving force) and whether the solution is stirred or quiescent (mass transfer).
Most experiments control the potential (volts) of an electrode in contact with the analyte while measuring the resulting current (amperes).
[7] To solve this problem, the roles of supplying electrons and providing a reference potential are divided between two separate electrodes.
Its only role is to act as reference for measuring and controlling the working electrode's potential and it does not pass any current.
The auxiliary electrode can be almost anything as long as it doesn't react with the bulk of the analyte solution and conducts well.
It is possible to run an experiment without a bulk electrolyte, but the added resistance greatly reduces the accuracy of the results.
A voltammogram (see linear sweep voltammetry) is a graph that measures the current of an electrochemical cell as a function of the potential applied.
To determine the concentration, values such as the limiting or peak current are read from the graph and applied to various mathematical models.
This can be observed by the maximum peak current (ip), and is identified by the highest point on the graph.
[10] To determine analyte concentrations, mathematical models are required to link the applied potential and current measured over time.
The Nernst equation relates electrochemical cell potential to the concentration ratio of the reduced and oxidized species in a logarithmic relationship.
It helps make predictions about how the forward and backward redox reactions affect potential and influence the reactivity of the cell.
The Tafel equation relates the electrochemical currents to the overpotential exponentially, and is used to calculate the reaction rate.
[11] The overpotential is calculated at each electrode separately, and related to the voltammogram data to determine reaction rates.
Where: The beginning of voltammetry was facilitated by the discovery of polarography in 1922 by the Nobel Prize–winning Czech chemist Jaroslav Heyrovský.
[23] Early voltammetric techniques had many problems, limiting their viability for everyday use in analytical chemistry.
Another problem included the residual current obtained from the charging of the large capacitance of the electrode surface.
[24] When Heyrovsky first recorded the first dependence on the current flowing through the dropping mercury electrode on the applied potential in 1922, he took point-by-point measurements and plotted a current-voltage curve.
In order to facilitate this process, he constructed what is now known as a polarograph with M. Shikata, which enabled him to record photographically the same curve in a matter of hours.
He gave recognition to the importance of potential and its control and also recognized the opportunities of measuring the limiting currents.
[25] In 1942, the English electrochemist Archie Hickling (University of Leicester) built the first three electrodes potentiostat, which was an advancement for the field of electrochemistry.
In the meantime, in the late 1940s, the American biophysicist Kenneth Stewart Cole invented an electronic circuit which he called a voltage clamp.
Lastly, there was also an advancement of preconcentration techniques that produced an increase in the sensitivity of the mercury electrodes.
A number of voltammetric systems are produced commercially for the determination of species that are of interest in industry and research.
[28] The determination of dissolved oxygen in a variety of aqueous environments, such as sea water, blood, sewage, effluents from chemical plants, and soils is of tremendous importance to industry, biomedical and environmental research, and clinical medicine.
One of the most common and convenient methods for making such measurements is with the Clark oxygen sensor, which was patented by L.C.