COVID-19 vaccine
According to studies published in 2005 and 2006, the identification and development of novel vaccines and medicines to treat SARS was a priority for governments and public health agencies around the world at that time.
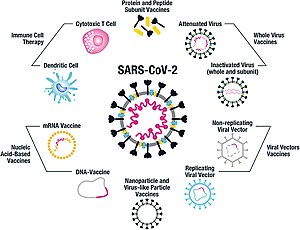
[45][46] However, other coronavirus proteins are also being investigated for vaccine development, like the nucleocapsid, because they also induce a robust T-cell response and their genes are more conserved and recombine less frequently (compared to Spike).
[43][50][51] Several of the synthetic vaccines use a 2P mutation to lock the spike protein into its prefusion configuration, stimulating an adaptive immune response to the virus before it attaches to a human cell.
[52] Vaccine platforms in development may improve flexibility for antigen manipulation and effectiveness for targeting mechanisms of COVID‑19 infection in susceptible population subgroups, such as healthcare workers, the elderly, children, pregnant women, and people with weakened immune systems.
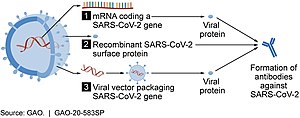
[66][67] The viral vector-based vaccines against COVID‑19 are non-replicating, meaning that they do not make new virus particles but rather produce only the antigen that elicits a systemic immune response.
[76] In August 2021, the developers of Sputnik V proposed, in view of the Delta case surge, that Pfizer test the Ad26 component (termed its 'Light' version)[77] as a booster shot.
Inactivated vaccines consist of virus particles that are grown in culture and then killed using a method such as heat or formaldehyde to lose disease-producing capacity while still stimulating an immune response.
[164] China provided low-rate loans to one vaccine developer through its central bank and "quickly made land available for the company" to build production plants.
[192] Its genetic sequence was published on 11 January 2020, triggering an urgent international response to prepare for an outbreak and hasten the development of a preventive COVID-19 vaccine.
[194][197][198][199] According to the Coalition for Epidemic Preparedness Innovations (CEPI), the geographic distribution of COVID‑19 vaccine development shows North American entities to have about 40% of the activity, compared to 30% in Asia and Australia, 26% in Europe, and a few projects in South America and Africa.
[205] The Pfizer–BioNTech partnership submitted an Emergency Use Authorization (EUA) request to the U.S. Food and Drug Administration (FDA) for the mRNA vaccine BNT162b2 (active ingredient tozinameran) on 20 November 2020.
[223] In November 2021, the full nucleotide sequences of the AstraZeneca and Pfizer/BioNTech vaccines were released by the UK Medicines and Healthcare products Regulatory Agency in response to a freedom of information request.
[237] On 10 December 2021, the UK Health Security Agency reported that early data indicated a 20- to 40-fold reduction in neutralizing activity for Omicron by sera from Pfizer 2-dose vaccinees relative to earlier strains.
[251] The US Centers for Disease Control and Prevention (CDC) recommends a fourth dose of the Pfizer mRNA vaccine as of March 2022[update] for "certain immunocompromised individuals and people over the age of 50".
Individuals who receive the combination of two different vaccines produce strong immune responses, with side effects no worse than those caused by standard regimens.
"[284] In one study, women who received both doses of a two-dose vaccine during the same menstrual cycle (an atypical situation) may see their next period begin a couple of days late.
[13] In January 2021, WHO Director-General Tedros Adhanom Ghebreyesus warned of problems with equitable distribution: "More than 39 million doses of vaccine have now been administered in at least 49 higher-income countries.
A human rights lawyer, Khaled Ali, launched a lawsuit against the government, forcing them to provide vaccinations free of charge to all members of the public.
International aid organizations have pointed at Nepal, Sri Lanka, and the Maldives, as well as Argentina, Brazil, and some parts of the Caribbean, as problem areas where vaccines are in short supply.
[333] A survey by The New York Times found that over a million doses of vaccine had been thrown away in ten U.S. states because federal regulations prohibit recalling them, preventing their redistribution abroad.
[335] To help overcome this problem, the Prime Minister of India, Narendra Modi, announced that they would make their digital vaccination management platform, CoWIN, open to the global community.
Around 142 countries, including Afghanistan, Bangladesh, Bhutan, the Maldives, Guyana, Antigua and Barbuda, St. Kitts and Nevis, and Zambia, expressed their interest in the application for COVID management.
MSF called for a Day of Action in September 2021 to put pressure on the WTO Minister's meeting in November, which was expected to discuss the TRIPS IP waiver.
However, in August, the United States government announced plans to offer booster doses eight months after the initial course to the general population, starting with priority groups.
[344][345][346] In September 2021, more than 140 former world leaders and Nobel laureates, including former President of France François Hollande, former Prime Minister of the United Kingdom Gordon Brown, former Prime Minister of New Zealand Helen Clark, and Professor Joseph Stiglitz, called on the candidates to be the next German chancellor to declare themselves in favor of waiving intellectual property rules for COVID‑19 vaccines and transferring vaccine technologies.
[347] In November 2021, nursing unions in 28 countries filed a formal appeal with the United Nations over the refusal of the UK, EU, Norway, Switzerland, and Singapore to temporarily waive patents for COVID‑19 vaccines.
[368] The Bureau of Investigative Journalism, a nonprofit news organization, reported in an investigation that unnamed officials in some countries, such as Argentina and Brazil, said that Pfizer demanded guarantees against costs of legal cases due to adverse effects in the form of liability waivers and sovereign assets such as federal bank reserves, embassy buildings, or military bases, going beyond what was expected from other countries, such as the US.
[370] On 13 December 2022, the governor of Florida, Ron DeSantis, said that he would petition the state supreme court to convene a grand jury to investigate possible violations in respect to COVID‑19 vaccines,[371] and declared that his government would be able to get "the data whether they [the companies] want to give it or not".
In a series of text messages to Paul Behrends, the close associate recruited for the Covaxx project, Prince described the profit-making possibilities of selling the COVID‑19 vaccines.
These have included exaggerated claims about side effects, misrepresentations about how the immune system works and when and how COVID-19 vaccines are made, a story about COVID-19 being spread by 5G, and other false or distorted information.