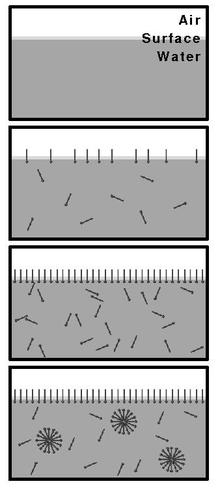
Critical micelle concentration
The value of the CMC for a given dispersant in a given medium depends on temperature, pressure, and (sometimes strongly) on the presence and concentration of other surface active substances and electrolytes.
[citation needed] For example, the value of CMC for sodium dodecyl sulfate in water (without other additives or salts) at 25 °C, atmospheric pressure, is 8x10−3 mol/L.
[citation needed] The common procedure to determine the CMC from experimental data is to look for the intersection (inflection point) of two straight lines traced through plots of the measured property versus the surfactant concentration.
[7][8][9] These fit functions are based on a model for the concentrations of monomeric and micellised surfactants in solution, which establishes a well-defined analytical definition of the CMC, independent from the technique.
In practice, CMC data is usually collected using laboratory instruments which allow the process to be partially automated, for instance by using specialised tensiometers.
Removal of oily soil occurs by modification of the contact angles and release of oil in the form of emulsion.
[citation needed] In petroleum industry, CMC is considered prior to injecting surfactant in reservoir regarding enhanced oil recovery (EOR) application.