Cryogenic gas plant
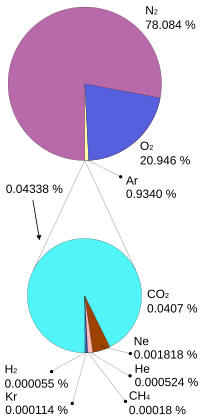
[1] As air is made up of nitrogen, the most common gas in the atmosphere, at 78%, with oxygen at 19%, and argon at 1%, with trace gasses making up the rest, cryogenic gas plants separate air inside a distillation column at cryogenic temperatures (about 100 K/-173 °C) to produce high purity gasses such as argon, nitrogen, oxygen, and many more with 1 ppm or less impurities.
The process is based on the general theory of the Hampson-Linde cycle of air separation, which was invented by Carl von Linde in 1895.
High purity liquid material such as oxygen or nitrogen produced by cryogenic plants is stored in a local tank and used as a strategic reserve.
Argon, xenon and helium are usually sold to customers in high pressure tank cars or trucks directly due to the smaller volumes.
[6] A cryogenic plant is composed of the following elements: Atmospheric air is roughly filtered and pressurised by a compressor, which provides the product pressure to deliver to the customer.
The process air enters the main heat exchanger in the coldbox where it is cooled in counter flow with the waste gas stream.
After passing a purity control valve, process air enters on top of the distillation column and flows down through the packing material.
It leaves the column and exits the cold box at ambient temperature through the main heat exchanger as a waste gas.