Cryptococcus neoformans
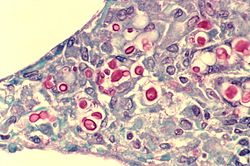
[8] The recognition of C. neoformans in Gram-stained smears of purulent exudates may be hampered by the presence of the large gelatinous capsule which apparently prevents definitive staining of the yeast-like cells.
In such stained preparations, it may appear either as round cells with Gram-positive granular inclusions impressed upon a pale lavender cytoplasmic background or as Gram-negative lipoid bodies.
[5] Studies suggest that colonies of C. neoformans and related fungi growing within the ruins of the Chernobyl Nuclear Power Plant may be able to metabolize ionizing radiation.
[14] Because it is normally a harmless soil fungus, C. neoformans must first adapt to its new environment inside the human body, making several virulent transformations, including the formation of a polysaccharide capsule.
[19][20] It has been speculated that this ability to manipulate host cells results from environmental selective pressure by amoebae, a hypothesis first proposed by Arturo Casadevall under the term "accidental virulence".
[21] In human infection, C. neoformans is spread by inhalation of aerosolized basidiospores or dehydrated fungal cells, and can disseminate to the central nervous system, where it can cause meningoencephalitis.
[23] Macrophages produce oxidative and nitrosative agents, creating a hostile environment, to kill invading pathogens.
[24] However, some C. neoformans cells can survive intracellularly in macrophages because of the protective nature of the polysaccharide capsule as well as its ability to produce melanin.
[23][3] Intracellular survival appears to be one of the factors contributing to latency, disseminated disease, and resistance to eradication by antifungal agents.
[25] However, precise mechanisms by which it passes the blood-brain barrier are still unknown; a 2014 study in rats suggested an important role of secreted serine proteases.
[28] Infections with this fungus were thought to be rare in people with fully functioning immune systems, hence C. neoformans is often referred to as an opportunistic pathogen.
[30] One benefit of meiosis in C. neoformans could be to promote DNA repair in the DNA-damaging environment caused by the oxidative and nitrosative agents produced in macrophages.
[29] Thus, C. neoformans can undergo a meiotic process, monokaryotic fruiting, that may promote recombinational repair in the oxidative, DNA-damaging environment of the host macrophage, and this may contribute to its virulence.
[citation needed] Infection begins in the lungs, and from there the fungus can disseminate to the brain and other body parts via macrophages.
Diagnosis methods include a serum cryptococcal antigen test and lumbar puncture with cerebrospinal fluid (CSF) examination to detect C.
[citation needed] A single high dose of liposomal amphotericin B with 14 days of flucytosine and fluconazole is recommended by the newest WHO guideline for cryptococcal meningitis.
[38] A new study found that brain glucose can trigger amphotericin B (AmB) tolerance of C. neoformans during meningitis which means it needs longer treatment time to kill the fungal cells.
Strikingly, Results of this study indicated that IPC synthase inhibitor aureobasidin A (AbA) can enhance the anti-cryptococcal activity of AmB.
[3] AmB is highly toxic to humans, and both fluconazole and flucytosine have been shown to cause development of drug resistanse in C. neoformans.