Smoothened
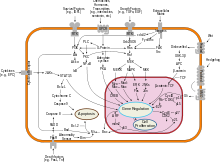
[11] The protein that carries the Hh signal across the membrane is the oncoprotein and G-protein coupled receptor (GPCR) Smoothened (Smo).
[12][13] Finding another method to target Smo activity in Hh-driven cancers would provide valuable information for novel therapeutics.
Cellular localization plays an essential role in the function of SMO, which anchors to the cell membrane as a 7-pass transmembrane protein.
[14] Vertebrate SMO that is mutated in the domain required for ciliary localisation often cannot contribute to hedgehog pathway activation.
[17] In invertebrates like Drosophila, SMO does not organize at cilia and instead is generally translocated to the plasma membrane following hedgehog binding to patched.
[22][23] Additionally, it is known that vertebrate SMO contributes to the activation of Gli as a transcription factor via association with ciliary structures such as Evc2, but these mechanisms are not fully understood.
[18] A leading hypothesis in the field is that Ptc regulates Smo by gating its access to cholesterol or a related sterol.
[25][26] Shh would work by inhibiting Ptc, which would increase accessible cholesterol concentrations and allow for the activation of Smo and transmission of the Hh signal.
[27] Additionally, Molecular Dynamics simulations suggest that vismodegib inhibits Smo through a conformational change that prevents cholesterol from binding.
[35] For example, oxysterol 20(S)-OHC is known to activate vertebrate SMO by binding the cysteine rich domain near its extracellular amino-terminal region.