Cyclophane
X-ray crystallography on '[6]paracyclophane' shows that the aromatic bridgehead carbon atom makes an angle of 20.5° with the plane.
[9] The proton NMR spectra of cyclophanes have been intensively examined to gain insights into the aromaticity of the benzene ring.
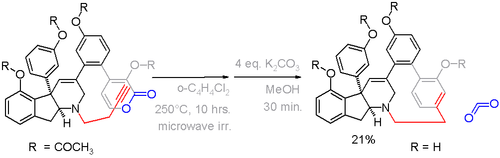
Shown below is the route to a [14][14]metaparacyclophane[11] in scheme 4[12] featuring a in-situ Ramberg-Bäcklund Reaction converting the sulfone 3 to the alkene 4.
Because of its potential application as an anticancer drug it is also available from total synthesis via an alkyne - pyrone Diels-Alder reaction in the crucial step with expulsion of carbon dioxide (scheme 5).
[16] These two classes of cyclophanes are both [7,7] paracyclophanes and were named after the species from which they were extracted: cylindrocyclophanes from Cylindrospermum lichenforme and nostocyclophanes from Nostoc linckia.
[17][18] One method for its preparation is by the 1,6-Hofmann elimination of 4-methylbenzyltrimethylammonium hydroxide:[19] The [2.2]paracyclophane-1,9-diene has been applied in ROMP to a poly(p-phenylene vinylene) with alternating cis-alkene and trans-alkene bonds using Grubbs' second generation catalyst:[20] The driving force for ring-opening and polymerization is strain relief.
Because the two benzene rings are in close proximity this cyclophane type also serves as guinea pig for photochemical dimerization reactions as illustrated by this example:[21] The product formed has an octahedrane skeleton.
First synthesized in 1967 by Stanley J. Cristol through the cycloaddition of anthracene and dibenzobarrelene,[22] the molecule has been used to study stacking and interactions between cations and pi orbitals, particularly with silver ions.



![Scheme 4. [14][14]metaparacyclophane](http://upload.wikimedia.org/wikipedia/commons/thumb/9/93/Metaparacyclophane.png/600px-Metaparacyclophane.png)