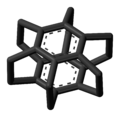
Superphane
Superphane is a 6-fold bridged cyclophane with all arene positions in the benzene dimer taken up by ethylene spacers.
The compound has been of some scientific interest as a model for testing aromaticity and was first synthesised by Virgil Boekelheide in 1979.
The benzene rings have been replaced by other aromatic units, such as those based on ferrocene or stabilized cyclobutadiene.
At each stage, two o-chloromethyl toluene structures are pyrolyzed to form o-xylylenes, either directly or via benzocyclobutene intermediates.
These structures were not isolated—they immediately react via [4+4] cycloaddition reactions to form two adjacent bridges between the aromatic rings.