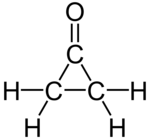
Cyclopropanone
Cyclopropanone is an organic compound with molecular formula (CH2)2CO consisting of a cyclopropane carbon framework with a ketone functional group.
[1] Cyclopropanone has been prepared by reaction of ketene with diazomethane[1][2] in an unreactive solvent such as dichloromethane.
In the presence of protic reagents such as carboxylic acids, primary and secondary amines, and alcohols, cyclopropanone converts to adducts, which are often isolatable at room temperature:[4][5] The C3O atoms are coplanar.
Cyclopropanones are intermediates in the Favorskii rearrangement with cyclic ketones where carboxylic acid formation is accompanied by ring-contraction.
[6] An oxyallyl intermediate is also proposed in the photochemical conversion of a 3,5-dihydro-4H-pyrazole-4-one with expulsion of nitrogen to an indane:[7] In this reaction oxyallyl intermediate A, in chemical equilibrium with cyclopropanone B attacks the phenyl ring through its carbocation forming a transient 1,3-cyclohexadiene C (with UV trace similar to isotoluene) followed by rearomatization.