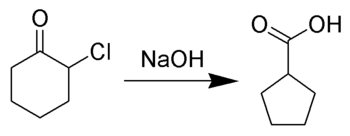
Favorskii rearrangement
In the case of cyclic α-halo ketones, the Favorskii rearrangement constitutes a ring contraction.
[6][7][8] The reaction mechanism is thought to involve the formation of an enolate on the side of the ketone away from the chlorine atom.
[9] The second step has also been proposed to be stepwise process, with chloride anion leaving first to produce a zwitterionic oxyallyl cation before a disrotatory electrocyclic ring closure takes place to afford the cyclopropanone intermediate.
When enolate formation is impossible, the Favorskii rearrangement takes place by an alternate mechanism, in which addition to hydroxide to the ketone takes place, followed by concerted collapse of the tetrahedral intermediate and migration of the neighboring carbon with displacement of the halide.
The photo-Favorskii reaction has been used in the photochemical unlocking of certain phosphates (for instance those of ATP) protected by p-hydroxyphenacyl groups.