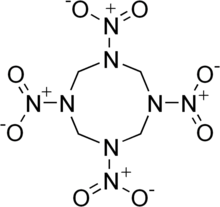
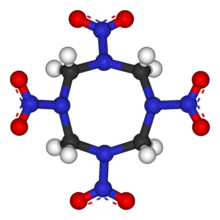
HMX
Because of its high mass-specific enthalpy of formation, it is one of the most potent chemical explosives manufactured, although a number of newer ones, including HNIW and ONC, are more powerful.
[3] HMX is used almost exclusively in military applications, including as the detonator in nuclear weapons, in the form of polymer-bonded explosive, and as a solid-rocket propellant.
Additionally, polymer-bonded explosive compositions containing HMX are used in the manufacture of missile warheads and armor-piercing shaped charges.
The HMX is built into a shaped charge that is detonated within the wellbore to punch a hole through the steel casing and surrounding cement out into the hydrocarbon-bearing formations.
[4][5] The Hayabusa2 space probe used HMX to excavate a hole in an asteroid in order to access material that had not been exposed to the solar wind.
At present, reverse-phase HPLC and more sensitive LC-MS methods have been developed to accurately quantify the concentration of HMX in a variety of matrices in environmental assessments.
Various acute and subchronic neurobehavioral effects have been reported in rabbits and rodents, including ataxia, sedation, hyperkinesia, and convulsions.