Cystathionine gamma-lyase
[1][2][3] Cystathionine γ-lyase also catalyses the following elimination reactions: In some bacteria and mammals, including humans, this enzyme takes part in generating hydrogen sulfide.
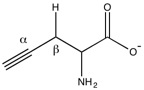
[6] Cystathionase uses pyridoxal phosphate to facilitate the cleavage of the sulfur-gamma carbon bond of cystathionine, resulting in the release of cysteine.
An additional deprotonation by a general base results in the formation of the external aldimine and removal of the lysine residue.
The basic lysine residue is then able to deprotonate the alpha carbon, pushing electron density into the nitrogen of the pyridine ring.
To reform the aldimine, this lone pair pushes back down, cleaving the sulfur-gamma carbon bond, resulting in the release of cysteine.
Lysine then attacks the external aldimine, pushing electron density to the beta carbon, which is protonated by a general acid.
[10]Excessive levels of H2S, due to increased activity of cystathionase, are associated with endotoxemia, acute pancreatitis, hemorrhagic shock, and diabetes mellitus.
The internal aldimine can regenerate, but the newly created vinyl ether sterically hinders the active site, blocking cysteine from attacking pyridoxal phosphate.