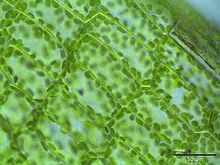
Cytoplasmic streaming
[4] This process is complicated, with temperature alterations in the system increasing its efficiency, with other factors such as the transport of ions across the membrane being simultaneously affected.
In plant cells, chloroplasts are transported within the cytoplasmic stream to optimize their exposure to light for photosynthesis.
[5] This rate of motion is influenced by several factors including light intensity, temperature, and pH levels.
One approach involves the introduction of Lugol's iodine solution, which effectively immobilizes the cytoplasmic streaming.
[citation needed] Alternatively, the compound Cytochalasin D, dissolved in dimethyl sulfoxide, can be employed to achieve a similar effect by disrupting the actin microfilaments responsible for facilitating cytoplasmic movement.
[8] As the myosin molecules "walk" along the actin filaments dragging the organelles with them, the cytoplasmic fluid becomes entrained and is pushed/pulled along.
[8][9] Chara corallina exhibits cyclic cytoplasmic flow around a large central vacuole.
[8] The flow of the cytoplasm in the cell of Chara corallina is belied by the "barber pole" movement of the chloroplasts.
Chloroplasts are never seen to cross these zones,[8] and as a result it was thought that cytoplasmic and vacuolar fluid flow are similarly restricted, but this is not true.
First, Kamiya and Kuroda, experimentally determined that cytoplasmic flow rate varies radially within the cell, a phenomenon not clearly depicted by the chloroplast movement.
[8] Further experiments on the Characean cells support of the Goldstein model for vacuolar fluid flow.
Further, recent experiments have shown that the data collected by Kamiya and Kuroda which suggested a flat velocity profile in the cytoplasm are not fully accurate.
Wild type versions of this plant exhibit cytoplasmic streaming due to the entrainment of fluid similar to Chara coralina, only at slower flow rates.
Resulting cytoplasmic flows rates are 4.3 microns/sec for the wild type and 7.5 microns/sec for the plants implanted with the rapidly moving myosin protein.
The plants implanted with human myosin Vb do not exhibit continuous cytoplasmic streaming.
[9] This suggests that the enhanced nutrient storage demonstrated by the Goldstein model allows for plants to grow larger and faster.
Cytoplasmic streaming in Chara corallina, however, enables chloroplasts to move around the stem of the plant.
[14] This intermittent exposure to photons due to cytoplasmic streaming actually increases the photosynthetic efficiency of chloroplasts.
In most plants, gravisensing requires a coordinated multi-cellular effort, but in Chara corallina, one cell detects gravity and responds to it.
[15] The barber pole chloroplast motion resulting from cytoplasmic streaming has one flow upward and another downward.
[8] The downward motion of the chloroplasts moves a bit faster than the upward flow producing a ratio of speeds of 1.1.
[15] Cytoplasmic streaming occurs due to the motion of organelles attached to actin filaments via myosin motor proteins.
[3] Thus, in Chara corallina, where motion of the chloroplasts and the myosin molecule follow a barber pole pattern, the actin filaments must all be similarly oriented within each section.
[3] In other words, the section where the chloroplasts move upward will have all of the actin filaments oriented in the same upward direction, and the section where the chloroplasts move downward will have all the actin filaments oriented in the downward direction.
Physarum polycephalum is a single-celled protist, belonging to a group of organisms informally referred to as 'slime molds'.
While, in humans, tropomyosin covers the site, only allowing contraction when calcium ions are present, in this amoeboid, a different molecule known as calmodulin blocks the site, allowing relaxation in the presence of high calcium ion levels.
Osmotic pressure gradients occur through the length of the cell to drive this cytoplasmic flow.
Modelling the fungal cells as a pipe separated at regular points with a septum with a hole in the center should produce very symmetrical flow.
[17] The eddies formed just before the septum allow for the formation of subcompartments where nuclei spotted with special proteins aggregate.
In other words, the random motion of the cytoplasmic particles create a net force toward the center of the cell.