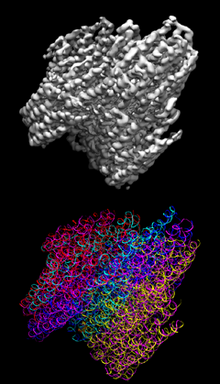
DNA origami
[9] On a chemical level, the hydrogen bonds that exist between the complementary base pairs provide strength and stability to the folded DNA origami structures.
One method of bypassing the need for a huge number of different strands is to use repeating units, which comes with the disadvantage of a distribution of sizes and sometimes shapes.
[9] To produce a desired shape, images are drawn with a raster fill of a single long DNA molecule.
[6] Bottom-up self-assembly methods are considered promising alternatives that offer cheap, parallel synthesis of nanostructures under relatively mild conditions.
The three primary methods of creating a dynamic DNA Origami machine are toehold mediated strand displacement, enzymatic reactions, and base stacking.
[12] While these methods are most commonly used, additional methods for creating dynamic DNA Origami machines exist, such as designing a directional component and using brownian motion to drive rotational movement of structures [13] or leveraging less commonly used DNA self-assembly phenomena like G-quadruplexes or i-motifs which can be pH sensitive.
One example application of creating dynamic structures is the ability to have a stimuli response resulting in drug release, which is presented by several groups.
[5] DNA is folded into an octahedron and coated with a single bilayer of phospholipid, mimicking the envelope of a virus particle.
[18][19] Researchers at the Harvard University Wyss Institute reported the self-assembling and self-destructing drug delivery vessels using the DNA origami in the lab tests.
[20] Researchers in the National Center for Nanoscience and Technology in Beijing and Arizona State University reported a DNA origami delivery vehicle for Doxorubicin, a well-known anti-cancer drug.
The DNA-Doxorubicin complex was taken up by human breast adenocarcinoma cancer cells (MCF-7) via cellular internalization with much higher efficiency than doxorubicin in free form.
The scientists theorized that the doxorubicin-loaded DNA origami inhibits lysosomal acidification, resulting in cellular redistribution of the drug to action sites, thus increasing the cytotoxicity against the tumor cells.
Further research has attempted to replace HIV antigens with SARS-CoV-2 and are testing whether vaccines show proper immune response from isolated B cells and in mice.
[26] Similarly, researchers from the Technical University of Munich have developed a method to have T-cells target tumor cells by using antigen coated DNA origami.
Meanwhile in vivo testing showed that their PTEs were capable of binding to the target proteins for several hours which validates the mechanism they designed.
[28] Many potential applications have been suggested in literature, including enzyme immobilization, drug delivery systems, and nanotechnological self-assembly of materials.
As a proof of concept, the team injected various kinds of nanobots (the curled DNA encasing molecules with fluorescent markers) into live cockroaches.
By tracking the markers inside the cockroaches, the team found the accuracy of delivery of the molecules (released by the uncurled DNA) in target cells, the interactions among the nanobots and the control are equivalent to a computer system.
[31][32] A research group at the Indian Institute of Science used nanostructures to develop a platform to elucidate the coaxial stacking between DNA bases.
Researchers at the National Institute of Chemistry in Slovenia are working on using rational design of protein folding to create structures much like those seen with DNA origami.